

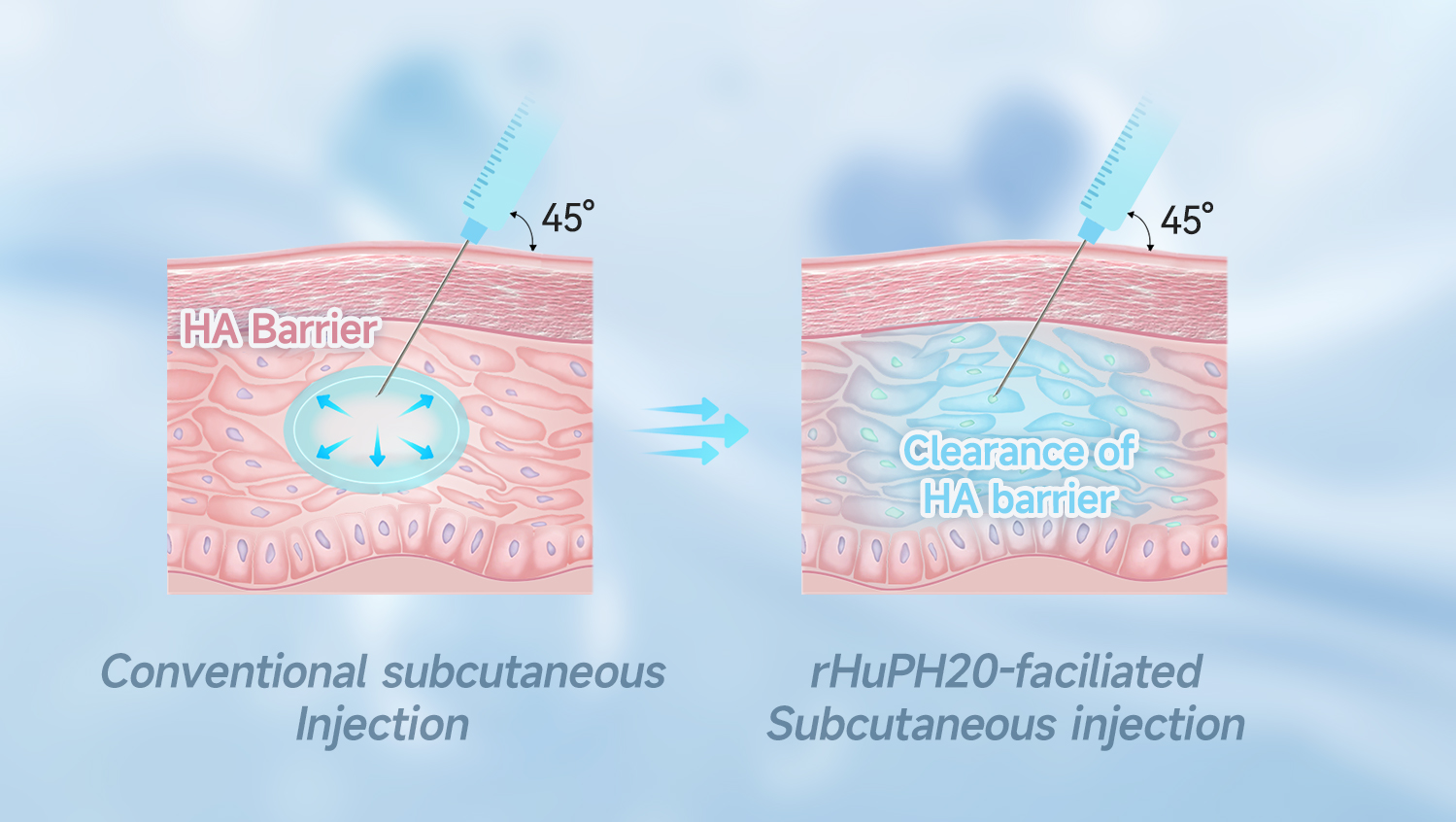
Our HYSORPTASE® is a recombinant human hyaluronidase (rHuPH20) synthesized in vitro using mammalian cells. It can locally degrade subcutaneous hyaluronic acid (HA), temporarily removing barriers to fluid flow, breaking through the subcutaneous injection volume limit of 2mL, and enabling the safe and efficient absorption of up to 1L of medication through the subcutaneous route. This improves patient experience, increases healthcare system efficiency, and enhances the competitiveness of partnered drugs.
By using HYSORPTASE® to develop co-formulations with biologics or small molecule drugs, co-formulated medications can overcome subcutaneous injection volume limitations, enabling rapid, convenient, and safe high-dose administration. This provides benefits to patients, healthcare systems, and manufacturers.
Several subcutaneous injection products using similar hyaluronidase co-formulation technology have been recognized in the global market.
HYSORPTASE® recombinant human hyaluronidase has data from hundreds of clinical uses.
Several co-formulated antibodies using HYSORPTASE® recombinant human hyaluronidase are in different stages of clinical research.

Bao Pharma develops and produces recombinant human hyaluronidase Hysorptase® in accordance with quality management requirements for recombinant drugs. The production process complies with current GMP standards, and the production scale meets commercial demand.
Regulatory Registration Information:
China Excipient Registration Number: F20240000658
FDA DMF Number: 041587

With Hysorptase® recombinant human hyaluronidase, it becomes possible to overcome the traditional volume limitations of subcutaneous injections, offering convenient subcutaneous therapies to patients in need, either in hospitals or at home.
Hysorptase® recombinant human hyaluronidase has undergone clinical validation and has been shown to effectively enhance the dispersion of drugs subcutaneously. Additionally, several co-formulation partnership projects are in various stages of development.
 Learn more
Learn more
